Mapping the different attributes of the shipping lane for a pharmaceutical can be one of the most important processes to ensure the safety and efficacy of the product. When shipping cold chain, an increased complexity is added.
Weather can vary month to month and extreme events such as snowstorms or heat waves create temperature excursion risks and delivery delays. Exploring how different shipping methods can create different thermal loads on a package and gathering real world temperature data in a lane can significantly reduce the risk of temperature excursions and ensure that lanes remain safe for pharmaceutical shipping.
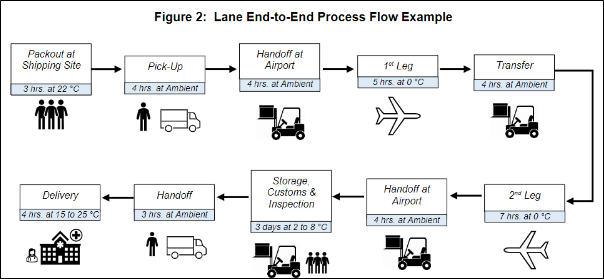
Shipping companies will provide different service levels such as ground shipping, 2-day guarantee, and white glove services that can create one of the largest impacts on the thermal load a package will experience during its transit.
Ground shipments create the largest variabilities in temperatures that a package experiences in transit and create a more weather-dependent lane. Shipments tend to be stored in warehouses or delivery trucks that are not temperature regulated during their transit where hot Summer days can create 50°C heat spikes and cold Winter nights can fall below the freezing point. In contrast, overnight and 2nd day shipments have a more predictable shipping lane due to the expedited nature of the shipment and will spend most of the shipment in environments that do not reflect weather data. An example of this can be seen when shipping from Arizona to Florida during the Summer months which has daily high temperatures around 45°C; the actual temperatures that the package experiences during its transit will typically be drastically lower than the weather data would indicate due to factors such as air transit which has temperatures down to 2°C in the cargo portion of the airplane and the expedited courier which keeps the package in temperatures closer to 20°C since it is not stationary for long durations.
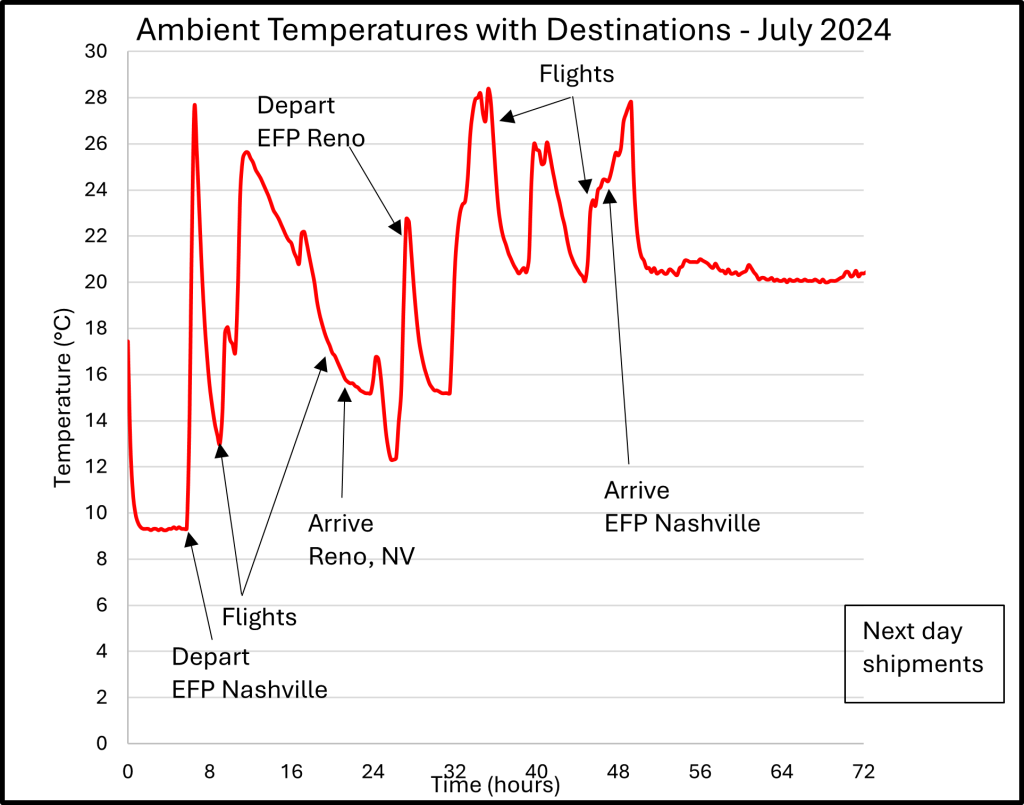
EFP develops methods for collecting accurate temperature data for your lane instead of relying on historical weather data. When performing a Performance Qualification (PQ) or during typical everyday shipments, a data logging device can be placed on the outside of your package to collect the temperature data that your package is experiencing during its transit. A secure location for the data logging device is typically directly under the top flaps of the outer carton of the cold chain packaging. This data can be crucial to ensuring that the ambient temperature profile used in a Design Qualification (DQ) or Operational Qualification (OQ) closely matches the real-world temperatures that the package will experience and assists with troubleshooting excursions in temperature.


While collecting temperature data in a lane, it is important to consider outside factors that can affect the temperatures being collected. Depending on the logger’s location on the cold chain packaging, any frozen materials such as gel packs or dry ice can drastically lower the temperature around the data logging device and record temperatures that are colder than the actual temperature the package is experiencing. To correct the cold deviations in the data set a few methods can be used such as using an oversized box with vent holes near the data logging device, shipping the data logging device in an empty box on the same lane as your cold chain package, or placing a data logging probe on the lid of your cold chain package during its DQ or OQ chamber testing then correlating the probe affixed to the lid with the actual chamber temperatures to see how the chilling effect changes over the duration of the shipment.



For more details on Lane Management for cold chain shipments or best practices for Performance Qualification of cold chain packaging, the newly released ISTA Pharma Committee documents (ISTA PHARMA-03 and ISTA PCW-01) provide informative resources for best practices and other nuances to consider. Please reach out to us here for more information.
For more details on Lane Management for cold chain shipments or best practices for Performance Qualification of cold chain packaging, the newly released ISTA Pharma Committee documents (ISTA PHARMA-03 and ISTA PCW-01) provide informative resources for best practices and other nuances to consider. Please reach out to us here for more information.

Written by Andrew Klasek, Product Engineering Manager.
Andrew began his career in the Environmental Chemistry field before coming to the packaging world where he currently runs the EFP Cold Chain Laboratory which develops shipping solutions for pharmaceutical and food shipments. Andrew also participates in the creation of whitepapers and industry guidance documents with the ISTA Pharma Committee covering topics such as developing temperature profiles for testing, lane testing, and simulation software.